1) So, this morning I promised a little tweetorial about variants and here we go. This will be from a vaccine/antibody point of view only, I won't comment on how infectious they are or if they are more pathogenic (I'll leave that to Boris Johnson 😉).
2) There are currently two variants for which there are insightful data sets. One is B.1.1.7 (aka the British variant) and the other one is B.1.351 (aka the South African variant). Let's start with B.1.1.7, which is easier. There is also more data. B.1.1.7 has a number of....
3)....mutations. Two are of special concern when it comes to vaccines since they might influence neutralizing epitopes. One is a change in position 501 (N501Y). This is located in the receptor binding domain of the virus were a lot of neutralizing antibodies bind. The other....
4)...one is a deletion in the NTD (Δ69/70) which may also affect antibody binding. So, let's look at the impact of those mutations. N501Y was actually identified early as mouse adaptive mutation when researcher passaged SARS-CoV-2 in mice. An RBD-based vaccine....
5)...already protected these animals providing the first hint that it might not be too problematic.
ncbi.nlm.nih.gov/pmc/articles/P…
6) @TheMenacheryLab kindly also provided data on Twitter right away showing that N501Y wasn't having a bad influence on neutralizing activity of human convalescent serum.
7) We also have nice data showing that both sera from individuals vaccinated with mRNA vaccines or from convalescent individuals have no issue with neutralizing a virus carrying N501Y.
medrxiv.org/content/10.110…
8) So, the last data points used actual used 'real' virus for neutralization. Pfizer also released data using a pseudotyped virus, which is a good surrogate. However, they looked at all mutations in B.1.1.7, not just the N501Y.
biorxiv.org/content/10.110…
9) Here are their results: Not much impact.
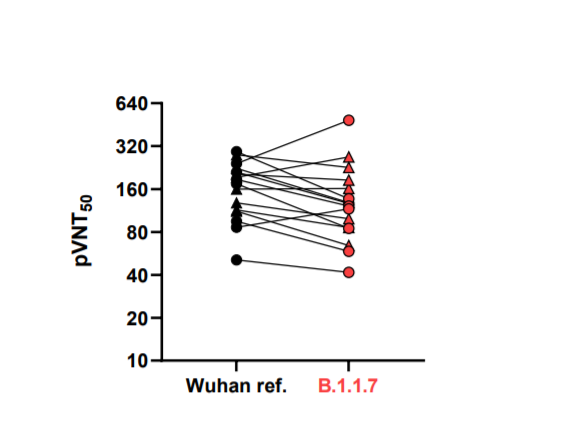
10) Rockefeller also had nice data showing some but only a small impact of N501Y on pseudtyped particle neutralization.
biorxiv.org/content/10.110…
11) And then there is the data from Moderna and the NIH Vaccine Research Center from today. This is also pseudotype particle neutralization with the full B.1.1.7 spike. Basically no impact.
biorxiv.org/content/10.110…
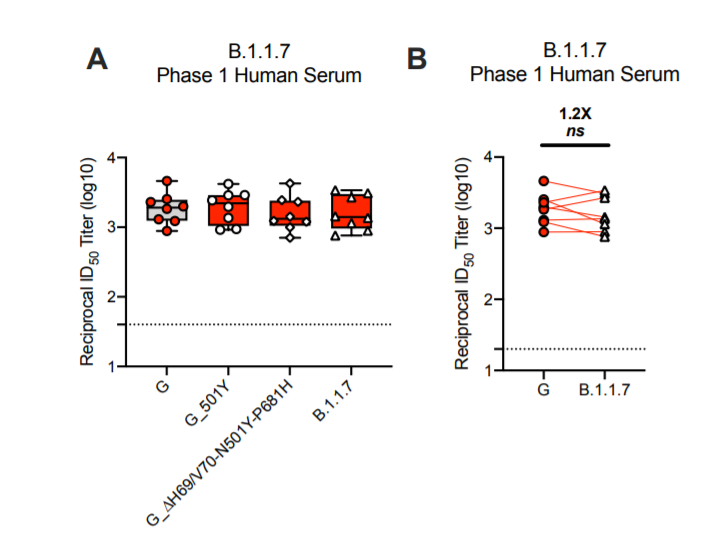
12) There is currently, to my knowledge, no good data set looking at neutralization of real B.1.1.7 virus by post-vaccination serum (I am sure some labs already have it 😉). However, I think we can draw relatively firm conclusions.
13) It does not look like B.1.1.7 will have a significant impact on vaccine-induced immunity. There might be some monoclonal antibodies that may not bind/neutralize anymore, but post-vaccination serum seems to do just fine.
14) So, before we continue with the more interesting variant B.1.351, I need to get a glass of Blaufränkisch (look it up, its delicious).
15) Let's continue. B.1.351 (also known as 501Y.V2) emerged in South Africa. It also has a number of mutations in the spike, including the N501Y we already know from B.1.1.7 but also other that are concerning including the RBD mutations K417N and E484K as well as...
16)....Δ242-244 which also happens to be a loop in the NTD, but a different one than in B.1.1.7. So, we know already that N501Y seems benign when it comes to neutralizing activity. The Δ242-244 may be an issue for NTD binding neutralizing antibodies (and there are additional....
17).....mutations in the NTD. But let's focus on E484K in the RBD firs, because that is the troublemaker. This mutation was identified early in studies looking at viral escape from mAbs, e.g. by groups at Rockefeller.
nature.com/articles/s4158…
18) Regeneron also picked it up.
science.sciencemag.org/content/369/65…
biorxiv.org/content/10.110…
19) Now, there are two papers from South Africa that looked at the impact of the full B.1.351 spike on neutralization. One is from Wibmer and colleagues using a pseudotyped particle assay and convalescent serum. Thee found a striking impact on activity.
biorxiv.org/content/10.110…
20) They found that many sera with low neutralization titers lost activity against a virus that only had the RBD mutations or all B.1.351 spike mutations. However, especially in subjects with higher titers some neutralizing activity was maintained. Interestingly.....
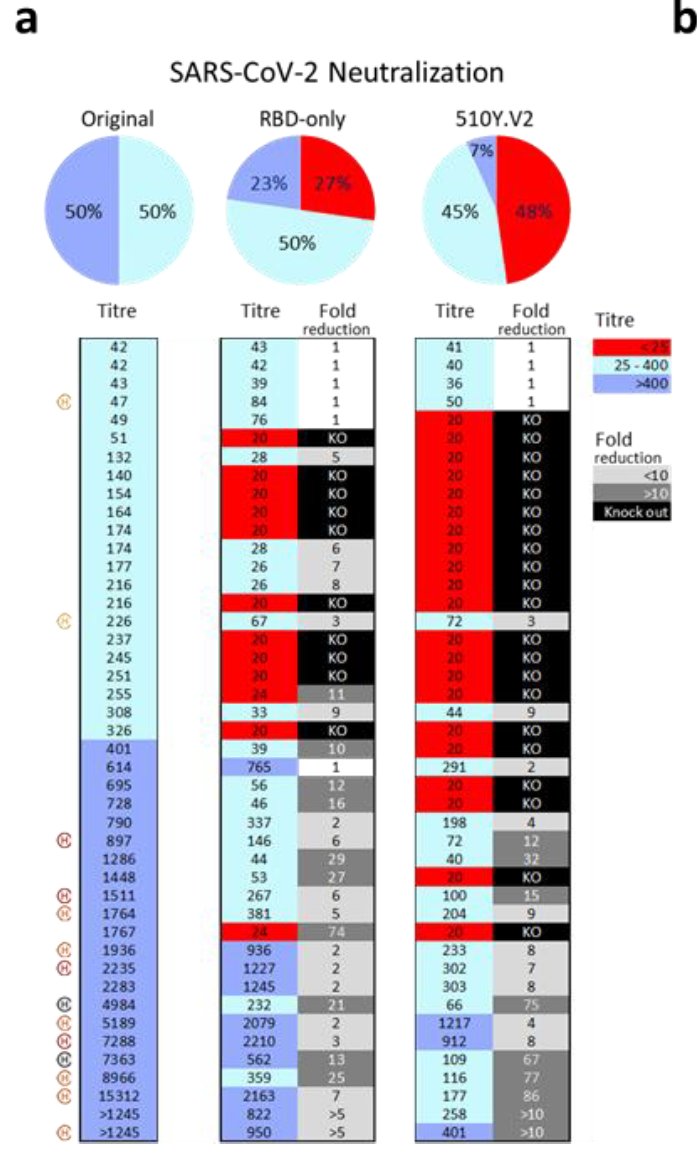
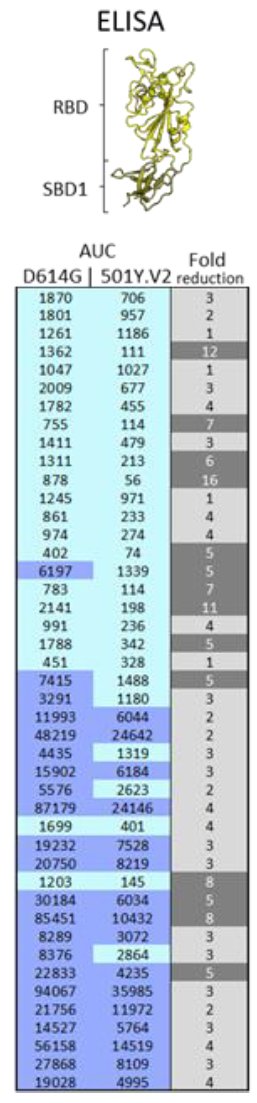
21)...there was less reduction in binding (ELISA) than in neutralization suggesting that maybe binding of antibodies to the spike is one component but perhaps higher affinity of the RBD to ACE2 could also contribute to this partial resistance.
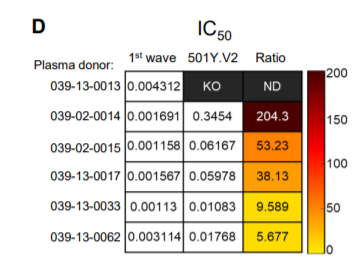
22) The second paper from Cele et al. looked at real virus. They also saw a significant reduction in neutralizing activity.
ahri.org/wp-content/upl…
23) Now, this is of course the point where people got really worried. So, how does this look for vaccines? Groups at Rockefeller gave us the first answer by looking at the three RBD mutations of B.1.351 in a pseudotyped particle neutralization assay.
biorxiv.org/content/10.110…
24) Here is their data. Just focus on the very right where the three mutations compared to wild type are show. This is with human vaccine serum (white=Moderna, red=Pfizer). There is a reduction but there is plenty of activity left.
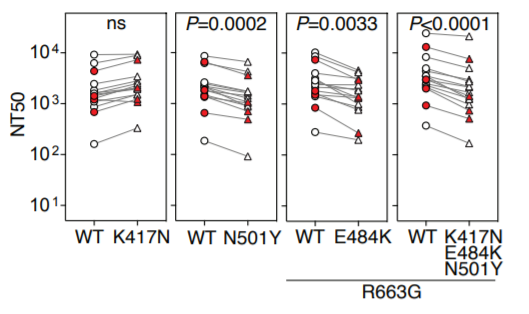
25) And then finally we are coming back to the data published by Moderna and the NIH Vaccine Research Center today. They have pseudotyped particle neutralization titers for the full B.1.351 spike in their preprint. The sera are from the Moderna mRNA-1273 vaccine trials.....
26) ....and they do see a reduction of about 6.4-fold. However, they start out with very high titers, leaving them with decent neutralization even against the B.1.351 variant.
biorxiv.org/content/10.110…
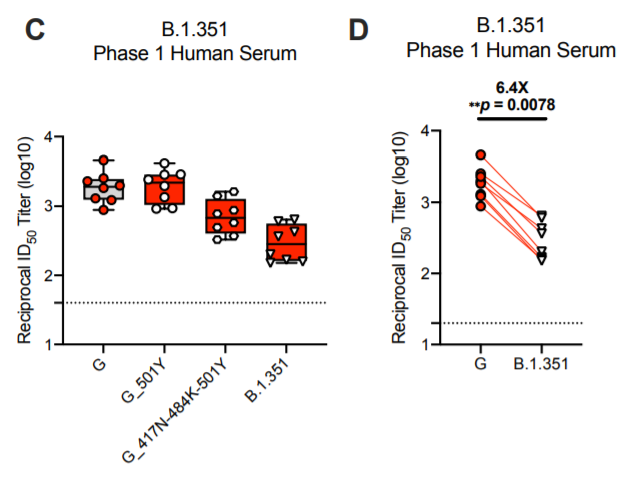
27) I expect the Pfizer sera would look the same in this assay, since the vaccines are almost identical and the immune responses are very similar.
28) We are certainly missing a lot of data here including neutralization data with the real B.1.351 virus. But believe it or not, it still takes a very long time to get vaccine sera or virus shipped from one country to another. So, paperwork is - as always - delaying important...
29)....research. Also, there is little data on B.1.1.248/P.1, also known as the Brazilian variant which has very similar mutations in the RBD compared to B.1.351. We certainly also need data with this variant.
virological.org/t/genomic-char…
30) Now, what does this all mean? It certainly is not the end of the world. mRNA vaccines induce very high neutralizing antibodies after the second shot (consistently in the upper 25-30% of what we see with convalescent sera). If that activity is reduced by 10-fold, it is....
31)...still decent neutralizing activity that will very likely protect. Furthermore, we know that the mRNA vaccines are already protective after the first shot when neutralizing antibody titers are low or undetectable in most individuals. This is another indication....
32)....that high titers are not needed and that a reduction in titer might be OK. However, it further highlights that giving one vaccination only (and follow with the second shot after a long interval) is risky business since it is less likely that those low titers....
33)....protect from a partially resistant variant. Another important factor is that binding antibodies seem to be affected less than neutralizing activity. Perhaps non-neutralizing antibodies also contribute to protection, e.g. via Fc-FcR interactions. For sure they seem to...
34)...be less impacted. It has also been shown that affinity maturation can help B-cells to deal with some of these variants, so that might help too. And then there are cellular immune responses. Memory B cells and T-cells have some time to respond to an infection since the...
35)...incubation time is relatively long. So that could help as well. In my opinion the worst case scenario is that the effectiveness of the mRNA vaccines will suffer a little but not too much. In the best case scenario there is not much of an effect.
36) However, it is important that high antibody titers are induced and not all vaccine candidates do that. The lower the initial titers, the higher the impact of these variants might be and I do find that worrisome. Now, what can be done? First, we need to do what every....
37)...good scientist is praying for a year now: We need to cut down on virus circulation. The more the virus replicates, the more infections there are the higher are the chances for new variants to arise. Also, we need to try and contain B.1.351 and B.1.1.248/P.1 as much....
38)...as possible. And then of course we need to prepare for making amendments to the vaccines if needed. Now, you hear from many that this is easy and can be done within weeks. It is not that easy. The devil is in the details. Yes, you can just change the vaccine, but....
39)...it is not the case that a new variant just takes over within a day and the old/wild type variants are gone. So, first you have to make sure that the new variant in the new vaccine also protects against the old variants. This experiment has not been done and it is likely....
40)...that there will be a mismatch in the other direction as well. If that is the case you need a bivalent vaccine. Or two different ones. The bivalent vaccine likely needs extended clinical trials again and that takes time. And who would want four shots if it is two....
41)...different vaccines? I actually like the idea of Moderna to give the first shot with the old variant and the second shot with the new variant. That might work, but will also need evaluation in clinical trials. Just to summarize: In general, I don't think there is a need to..
42)..panic. I think the mRNA vaccines and other vaccines that give high titers (Novavax etc.) will still work nicely. But this is a wake-up call! And I do worry about global vaccine supply since many vaccines for the global market induce lower neutralization titers.
43) And remember: Everybody can contribute to avoid the emergence of variants by not getting infected and by not passing on the virus to other. Now I'll finish my Blaufränkisch. Have a good night.
